
Corrosion is a common problem. Why does this happen?


Reason:
Water has soluble salts and minerals which are responsible for the water’s conductivity and PH.
Hard waters – like water from underground have TDS levels more than the pond water. Normally the municipal water supply is treated water acquired from Lakes and Ponds or Reservoirs. These sources have rainwater stored hence the water is already distilled and have less salts and minerals.
Whereas the underground water lays deep in the ground and comes in contact with many minerals and salts which tend to dissolve in the water and makes it hard.
Let’s not stray from the topic more…
And take a look at the phenomenon of electrolysis:

Electrolysis
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by electrolysis.
When some voltage is applied to the probes laying in water, the salts and minerals get charge and tend to get attracted towards the probe or terminal.
This causes a layer of salts and minerals broken to a hard layer of coral like structure as shown in the figures above.
And you can’t avoid it.
Electrolysis can only happen if the water is conductive.
Although pure H2O is not conductive but there is no way we can consume pure water.… It will make us sick.
Okay, … But What can be done?
First thing one can think of that why do we need conductivity to sense anyways!!
Yes, your point is correct. There are other ways to do it, but those are all mechanical switches we are talking about and face other challenges. They are called float switches; you may find their working here:
https://instrumentationtools.com/float-level-switch-working-principle-animation/
But again, they have their own challenges. Since they are mechanical sensors, they get jammed after some time, especially in hard waters.
We don’t want to get out of one problem just to get into another. Right?
What do we do now?
We at Walnut Innovations did a lot of juggles which you might not be interested. The extract of that is we used alternative current for sensing.
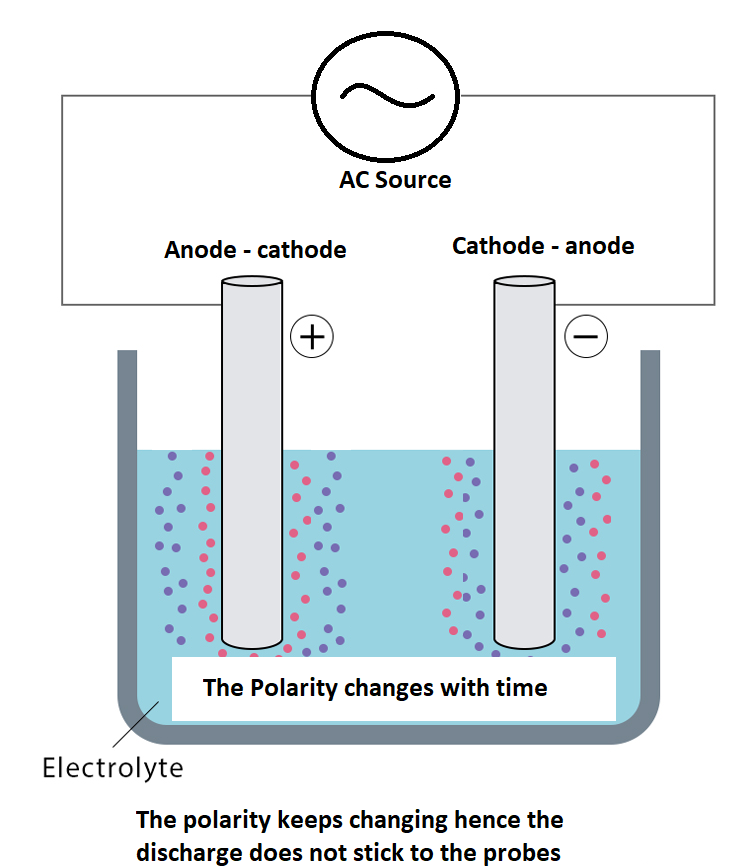
Non Electrolytic sensing
No, there is no way we can escape electrolysis while we are sensing by conductivity.
However, we can reduce the effect if we switch the positive and negative terminal’s polarity with the same frequency and intensity.
It can be done easily by applying an alternative current of small voltage.
The ions or say the chemicals charged will keep moving in between and won’t stick to any charge, they will just keep roaming too and fro.
This way we can keep the property of water intact and do the conductivity sensing at the same time.
Wrapping up..
There are a lot of Water level indicators and Controllers in market. My advice it to buy the ones with AC sensing probes. These will cost a bit more but are worthwhile.
You won’t need to scratch and clean the probes every month then.
Please visit our water level controllers
Walnut Innovations Water level Controller Series
All of them are Electrolysis Free products and been endorsed by thousands of users for a decade.
I will post a few more articles on other problems and solution of Water level Controllers which are faced by purchasing cheap controllers.
Please view, share and comment.



Hi, this is a comment.
To get started with moderating, editing, and deleting comments, please visit the Comments screen in the dashboard.
Commenter avatars come from Gravatar.
I need to to thank you for this good read!! I certainly loved every bit of it. I have you bookmarked to check out new stuff you postÖ
Thanks for the endorsement, though more of my time spends on development, I will post more when I get time.